John Dalton (1766 - 1844)
Dalton's Elements and Compounds (1808, 1810,1827)
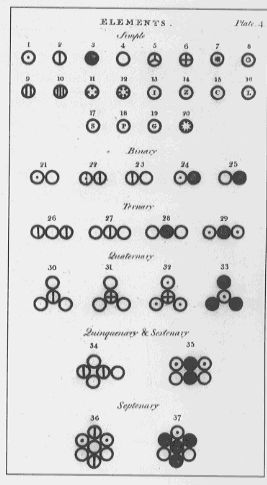
number 21 is water, HO
Dalton's atomic theory:
matter consists of atoms
atoms can not be created or destroyed
all atoms of the same element are identical
different elements have different types of atoms
chemical reactions involve rearrangements of atoms
compounds consist of "compound atoms" (molecules) composed of atoms from the constituent elements
Problems:
assumed all gases were monoatomic, e.g. oxygen is O
assumed simplest compounds were binary, e.g. water is HO
his atomic weights were approximate
no knowledge of isotopes