
Ernest Rutherford (1871 - 1937)

J. J. Thomson (left) & Rutherford (right) in 1938
His experiment used alpha particles (1910-11)
(carried out by H. Geiger & E. Marsden)
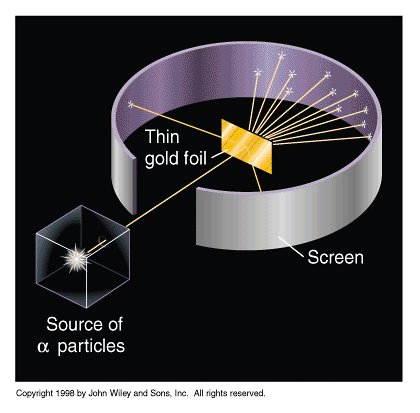
A few alpha particles (1 in 8000) were reflected back at the source.
"...as if you fired a 15-inch shell into a piece of tissue paper and it came back and hit you."
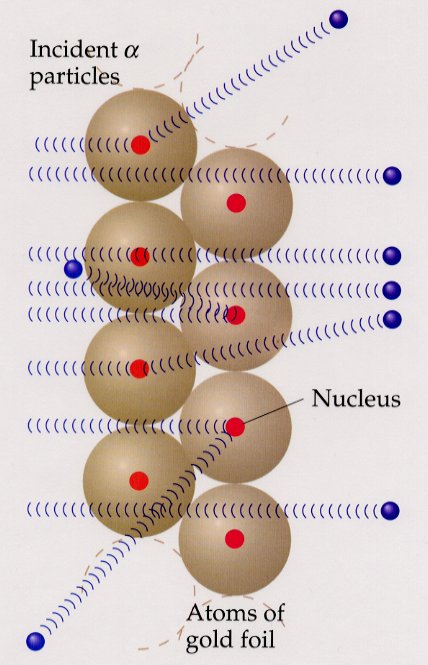
The nuclear atom: a small, heavy, positively charged nucleus surrounded by space in which electrons move.
If the nucleus were the size of a golf ball, the atom would be about 3 miles across.