Charles (1746-1823)
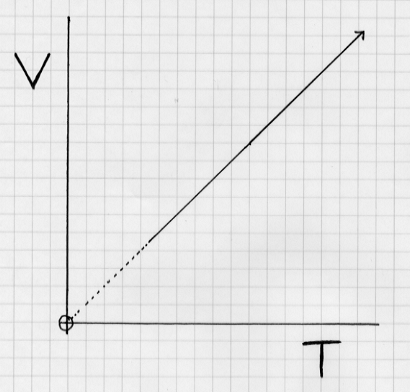
Charles' Law: the volume of a gas varies directly with temperature.

As the temperature increases, volume increases. Pressure is assumed constant.
Pressure and temperature have a similar direct relationship, when volume is constant.