The
Elements of the Uncertainty Principle
Physicists have defined many quantities with which to describe properties of an object. Two of these are important to the Heisenberg uncertainty principle. The first of these is the position of an object. This is fairly straightforward. Just pick a point, and measure how far left or right of that point the object is. That number is your position. In three dimensions, it takes three numbers to describe the position of an object: how far east or west, how far north or south, and how far above or below a point the object is.
The
other quantity is called momentum. Momentum is a measure of how
"hard" an object is moving. It's the product of the "mass"
(think "weight") of an object and its velocity. (If an object is
moving close to the speed of light, the formula is a bit different.) Think of
it this way: if a baseball is lobbed gently at you, it doesn't hurt as much as
it does if it strikes you at 90 mph. And a ping-pong ball that hits you at 20
mph doesn't hurt nearly as much as a bowling ball that hits you at the same
speed. So how "hard" an object is moving depends on both the velocity
of the object and its mass. This is reflected in the definition of momentum.
These
two quantities, position and momentum, are the subject of the Heisenberg
uncertainty principle. The principle describes the precision with which these
two quantities can be known for any object.
Wave-particle
duality: light
Light
as a wave
The story of quantum physics probably best begins with light. In the early days of physics (say, before the nineteenth century) about the only things people knew about light was that it was bright, it was fast, and it came in a variety of colors. Very little was known about the nature of light, and one of the great debates about light was over the question of whether light was made of a bunch of "light particles," or whether light was a wave. Around 1800, a man named Thomas Young apparently settled the question by performing an experiment in which he shone light through very narrow slits and observed the result. Here's the idea behind it.
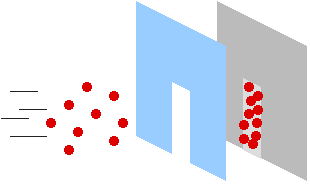
Suppose
you have a whole bunch of ping-pong balls. You stand back about fifteen feet
from a doorway, and one by one you dip the balls in paint and throw them
through the door, at a wall about 5 feet past the door. Well, you'll get a
bunch of colored dots on the wall, scattered throughout an area the same shape
as the door you're throwing them through. This is how particles (such as
ping-pong balls) behave.
On
the other hand, waves don't behave this way. Think of water waves. When a wave
encounters an obstacle, it goes around it and closes in behind it. When a wave
passes through an opening, it spreads out when it reaches the other side. And
under the right conditions, a wave passing through an opening can form
interesting patterns on the other side, which can be deduced mathematically.
So
here's what Young did. He took light, and shone it through a very narrow slit,
and then shone that light through two very narrow slits, very close
together. He then observed the result of this on a screen. Now if light is made
up of particles, then the particles should pass straight through the slits and
produce two light stripes on the screen, approximately the same size as the
slits. (Just like the ping-pong balls in the picture above.) On the other hand,
if light is a wave, then the two waves emerging from the two slits will
interfere with each other and produce a pattern of many stripes, not
just two. (Trust me on this, or I'll be forced to go through the math...)
![]()
The
result? Young found the interference pattern with many stripes, indicating that
light is a wave.
Later
in the nineteenth century, James Clerk Maxwell determined that light is an electromagnetic
wave: a wave of oscillating electric and magnetic fields. When Heinrich Hertz
experimentally confirmed Maxwell's result, the struggle to understand light was
finished. Case closed.
Light
as particles
Case reopened. In the early years of the twentieth century, the science of physics saw an upheaval of a magnitude unseen since the time of Isaac Newton. This upheaval is often said to have started with Max Planck. Planck was considering the problem of "black body radiation." You're familiar with the fact that very hot objects glow. (Charcoal briquets, light bulb filaments, and the sun, for example.) Well, an object doesn't have to be hot in order to emit this glow. Any object, regardless of temperature emits a glow. The difference is that for objects at ordinary temperatures the glow is much dimmer than for hot objects, and is predominantly infrared light, which we can't see. Anyway, Planck was considering, as others had done, the spectrum (the different colors produced and their relative intensities) of the light emitted by a perfectly black object at different temperatures; that is, black body radiation.
The
problem was that physicists had already applied the best of all their knowledge
about thermodynamics and electromagnetism to this problem, and they didn't like
the result. The result was that if you examined the spectrum from infrared
light, through the visible spectrum (red through violet), and on into
ultraviolet light, the intensity of the emitted light just goes up and up and
up. This means that an object at any temperature (even if not completely black)
radiates an infinite amount of energy, a result that clearly makes no
sense, and was termed the "ultraviolet catastrophe."
In
1900 Planck discovered a way around this problem. He found that if light can
only be emitted in small bursts, rather than a continuous wave, the
resulting calculation not only made sense, but matched what people measured
experimentally.
Albert
Einstein carried Planck's discovery one step further when he applied it to
another nagging inconsistency in physics, this one concerning the
"photoelectric effect." When light is shone on a metal surface,
electrons can be ejected from that surface. This is called the photoelectric
effect. Without going into detail, if one assumes that light is a wave, as
Young showed, then there are certain features of the photoelectric effect that
simply seem impossible. What Einstein showed is that if one assumes that light
is made up of particles (now called "photons"), and if these
particles have the properties described by Planck for his small bursts of
light, then the photoelectric effect all makes sense. When Robert
Millikan performed experiments which confirmed some of Einstein's ideas,
Einstein won the Nobel Prize (as did Millikan a couple years later).
Wave-Particle
Duality
So what's the answer? Is light a wave, or is light a flow of particles? Well, the bottom line is that it's neither one. Light is are you ready? a "quantum vector field." That phrase doesn't give you much of a mental picture, does it? I actually kind of know what a quantum vector field is, and it doesn't give me any mental picture. The fact is that the true nature of light defies mental picturing, because it's not quite like anything we can lay our hands on. Under certain conditions, such as when we shine it through narrow slits and look at the result, it behaves as only a wave can. Under other conditions, such as when we shine it on a metal and examine the spray of electrons that comes off, light behaves as only particles can. This multiple personality of light is referred to as "wave-particle duality." Light behaves as a wave, or as particles, depending on what we do with it, and what we try to observe. And it's wave-particle duality that lies at the heart of the Heisenberg uncertainty principle.
Wave-Particle
Duality: Electrons
And so something that physicists had long considered to be simply a wave, light, turned out to behave like particles. The next question, asked by physicist Louis de Broglie, was "If waves can behave like particles, can particles behave like waves?" In the case of light, exposing the particle properties was simply a matter of creating the right circumstances (such as the photoelectric effect). The right circumstances for observing wavelike properties of electrons was created by physicists Davisson and Germer.
These
guys aimed a beam of electrons at a crystal, and observed the electrons that
were reflected off it. Now a crystal consists of atoms arranged in nice
straight rows (or some other very orderly pattern). If the electron beam can
behave as a wave, then as the wave is reflected off the crystal, the rows of
atoms should have the same effect as the slits in Young's experiment. The
result is that instead of the electrons being scattered from the crystal
randomly, the reflected electrons exhibit an interference pattern like the
light in Young's experiment.
Anyway,
Davisson and Germer did the experiment, and this is exactly what they found.
The electron beam was reflected like a wave, rather than like particles. In
other words, they found, as de Broglie had speculated, that waveparticle
duality is a property not only of light (photons), but of matter as well.
Electrons, protons, alpha particles, and anything else that physicists might
discover.
Now
de Broglie's theory was more than just qualitative. De Broglie derived the
mathematical relationships between the particle properties of a waveparticle,
and its wave properties. When a physicist thinks of a wave, this is what he or
she sees:

The distance between the top of one wave and the top of the next (about two inches in the graphic above) is called the wavelength of the wave. If you pick a point on the graph above and count the waves as they go by, the number of waves per second is called the frequency of the wave. Planck derived a relationship between the frequency of a wave and the kinetic energy of the corresponding particle. (Like momentum, kinetic energy is a measure of how "hard" a particle is moving.) And de Broglie derived a relation between the momentum of a particle and the wavelength of the corresponding wave.
The greater the momentum, the shorter the wavelength.
The greater the kinetic energy, the higher the frequency.
This holds true for any atomic or subatomic particle, and in principle it holds for larger objects too, except that the wave properties of macroscopic objects are pretty much impossible to detect.
The
Meaning of the Wave
At this point we have a bit of a mystery. We've seen that electrons, under some circumstances will behave like particles, and under other circumstances will behave like a wave. But a wave of what? A wave implies that something is oscillating. In a water wave, the water itself goes up and down. In a sound wave, air pressure oscillates as the wave passes through. In a light wave, there's an electric and magnetic field that oscillate, as Maxwell and Hertz showed. With an electron wave, there's got to be something oscillating, but what?
To
answer this, let's go back and reconsider Young's experiment. Stripping away
the details, we shine light through a couple of slits, we place a screen behind
the slits to observe the result, and an interference pattern ![]() appears
on the screen. The screen is brightest where the amplitude of the wave
striking the screen is greatest, and where the amplitude is small, the screen
is dim. (Amplitude is a word physicists use to describe the size of the
oscillations in a wave. For example, in a water wave, the amplitude refers to
the height of the wave. In a light wave, the amplitude refers to the size of
the fluctuations in the electric field, etc.)
appears
on the screen. The screen is brightest where the amplitude of the wave
striking the screen is greatest, and where the amplitude is small, the screen
is dim. (Amplitude is a word physicists use to describe the size of the
oscillations in a wave. For example, in a water wave, the amplitude refers to
the height of the wave. In a light wave, the amplitude refers to the size of
the fluctuations in the electric field, etc.)
Now
let's think of this in terms of particles (photons). The photons pass through
the slits and strike the screen. (Then they bounce off in all directions; some
of them reach our eyes, and so we're able to see that the screen is lit up.)
The bright stripes on the screen are bright because lots of photons are hitting
there, while places where very few photons strike will be dim.
Finally,
let's consider a single photon. It passes through the slits and strikes the
screen. Where is it going to hit? Well, they're hitting all over the place, so
we really can't tell for sure where this one is going to go. On the other hand,
we can state with confidence that it will probably hit one of the places
on the screen where it's bright, since that's where most of them are
hitting. (That's why it's bright, remember?) And the brightness of the light on
the screen depends on the amplitude of the light wave at that point. (To be
more specific, the brightness depends on the square of the amplitude.)
So
we've linked the amplitude of the wave to the number of photons in that area,
and the number of photons in an area to the probability of any given
photon being in that area. This is how the wave and the particle are related in
waveparticle duality. The amplitude of the wave tells us the probability of a
particle being there. We can think of the wave as a wave of probability.
So
Young's experiment works like this. Suppose instead of a whole beam of light,
we launch just a single photon through the slits and observe where it hits the
screen. From the time the photon passes through the slits, we don't know where
it is until we "measure" its location by letting it hit the screen.
In the meantime, the wave travels from the slits to the screen, and the
amplitude of the wave determines the probability of where we'll find the photon
when we do measure its location. I've put this last bit in bold
print, because it's a central fact of waveparticle duality, whether we're
dealing with photons, electrons, neutrinos, or whatever.
From
Many Waves, One
(I'm assuming you can stop these waves somehow with your browser once you've taken in the motion. If you can't, I'm sorry for the distraction.*)
The
mathematical prototype of a wave,

is shown here only in part. It really extends infinitely far to the left and
right. But in quantum physics this wave is supposed to describe an electron (or
some other waveparticle). Now a subatomic particle is a small thing and
typically isn't spread out over the entire universe. So the perfect wave above,
while possessing the virtue of mathematical simplicity, doesn't really describe
what we think of as a particle. A more appropriate wave might look something
like this:
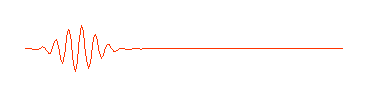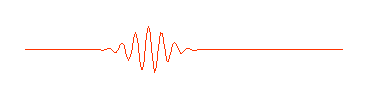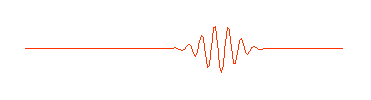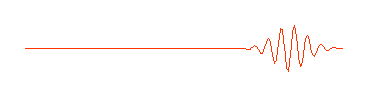
Physicists call this a "wave packet." Now remember, that the
amplitude (size) of the wave in any region determines the probability of
finding the particle there. If we can perform an experiment which would
actually reveal where the particle is, we would be more likely to find it near
the center of the wave packet, where the amplitude of the wave is largest,
rather than out near the ends of the packet, and we certainly wouldn't expect
to find it out beyond the ends of the wave packet. The particle is somewhere in
the wave packet, but we don't know exactly where.
Next,
let's consider the momentum of this waveparticle. De Broglie, Davisson, and
Germer showed that the momentum of the particle is related to the wavelength of
the wave (the distance between successive peaks in our wave, remember). For the
wave at the top of the page, that extends across the entire universe, this is
not a problem. But for the wave packet below it, the question of wavelength is
not so simple. The distance from one wave to the next is not exactly the
same everywhere, and beyond the wave packet, where the wave dies out, the whole
idea of wavelength becomes a bit hazy.
It
turns out, however, that this problem was worked out mathematically by Jean
Baptiste Joseph Fourier, early in the nineteenth century. To illustrate
Fourier's discovery, here's a rather strange looking wave:
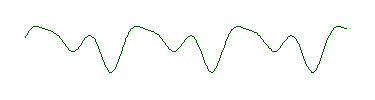
It doesn't have the shape of the "perfect" wave at the top of the
page. However, this wave is in fact the sum of these three waves:
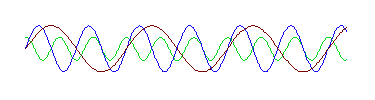
That is, if we take these three waves and add them up, we get the strange wave.
And notice that each of these three waves has the same shape as the
"perfect" wave prototype at the top of the page. In other words, the
strange wave does not have a single wavelength, but is made up of waves with
three different wavelengths. (If you have some graphing software, even a
spreadsheet, you can verify this for yourself. The three perfect waves are
described mathematically by sin(x), sin(2x) and .5cos(3x), and you can verify
that the strange wave is the sum of these three functions.)
What
Fourier showed was that any wave, no matter how bizarre can be described
as a sum of perfect waves with different amplitudes and wavelengths. It may
take an infinite number of these perfect waves, but it can be done.
So
the wave packet shown near the top of this page, which could represent the wave
of an electron for example, doesn't have a single wavelength. It's a wave with
a combination of lots of different wavelengths. And this fact is what leads to
the Heisenberg uncertainty principle.
The
Heisenberg Uncertainty Principle
Let
me sum up what I've said so far. The microscopic world has a property called
"waveparticle duality." (So does the macroscopic world, but the
effects of waveparticle duality aren't noticeable for large objects.) What
this means is that every particle, such as a photon, electron, proton,
positron, and so on, has a wave associated with it. The amplitude (size) of
this wave describes the probability of finding the particle in that region. In
any observation of the particle, it will probably be found where the amplitude
is large, and will probably not be found where the amplitude is small, but
there's an uncertainty in the position of the particle.
The
wave will generally not be spread throughout the entire universe, but will be
confined to a small region in space. This "localized" wave is called
a wave packet. A wave packet does not have one specific wavelength, but is made
up of waves of a variety of wavelengths. And the momentum of a waveparticle
depends on its wavelength. So a waveparticle that isn't spread out over the
entire universe doesn't have a specific momentum; it has many momenta. In other
words, there's an uncertainty in the momentum of the particle. (We're getting
close, can you tell?)
Now
suppose the wave is pretty much confined to a region in space with a width
we'll call Dx. (For you statheads out there, this is the standard deviation of
the square of the wave function. The "wave function" is just the
mathematical expression that describes the wave.) And suppose the momenta that
make up the wave fall within a range given by Dp (again, a standard deviation
of the momentum distribution). These two numbers represent the uncertainty in
the position of the particle and the uncertainty in the momentum of the
particle.
Which
brings us to the uncertainty principle. What Heisenberg discovered is that a
wave packet confined to a very small region must be made up of a lot of
different wavelengths, and therefore a lot of different momenta. In other
words, if the uncertainty in the position of the particle is small, the
uncertainty in the momentum is large. And similarly, a particle whose wave
packet is made up of only a few wavelengths (and hence only a few momenta) will
be spread out over a large region. That is, if the uncertainty in momentum is
small, the uncertainty in position is large.
Mathematically,
Heisenberg's result looks like this:
![]()
This
means just what I said in the preceding paragraph. The smaller Dx is, the
larger Dp has to be (and vice versa) so that the product is larger than
h-bar/2. H-bar (the h with a slash through it) incidentally, is called Planck's
constant. It first turned up in Planck's paper on blackbody radiation.
Now
the uncertainty principle is not something we notice in everyday life. For
example, we can weigh an automobile (to find its mass), and all automobiles
have speedometers, so we can calculate the momentum. But doing so will not make
the position of the car suddenly become hazy (especially if we're inside it).
So measuring the momentum of the car seems to produce no uncertainty in the
car's position.
The
reason we don't notice the uncertainty principle in everyday life is because of
the size of Planck's constant. It's very small:
![]()
If
you don't know what a Joule·second is, don't worry about it. The point is that
Planck's constant is a very small number! So let's take an example. The
mass of a baseball is about .145 kg. Let's suppose a radar gun can measure its speed
to within .1 mph. (I'm told by one reader that it's actually more like .25 mph,
but I've already worked it out for .1. Either way the result is about the
same.) This .1 mph (which is about .045 meters/second) is the uncertainty in
the velocity of the ball. Since momentum is mass times velocity, this means the
uncertainty in the the momentum of the ball is .145 x .045 = .0065 kg·m/s.
Then
the Heisenberg uncertainty principle tells us that the uncertainty in position
has to be at least as large as:
![]()
which
comes out to 8 x 10-30 (that is, .000000000000000000000000000008)
millimeters. Well, you can't measure that, especially for a moving baseball, so
you never notice the uncertainty in position. You get a similar result when you
apply the uncertainty principle to any object large enough to see. The
uncertainty is just too small to be noticed. While the uncertainty principle
applies to anything, it's only noticeable for very microscopic particles. In
the physics of subatomic particles, it's an often crucial fact that we can't
know both the position and the momentum of a particle. That's the Heisenberg
uncertainty principle.
The
Copenhagen Interpretation
"Those who are not shocked when they first come across quantum theory cannot possibly have understood it." ---Niels Bohr
Anyone who has studied quantum physics will agree that it's very strange. Not only do we not know everything we would like to know about a particle, but we can't know everything we would like to know. This is what the uncertainty principle tells us. But there are many areas of science that are strange, and this is not the most unique feature of quantum physics. What makes quantum physics truly special is this: Even if we know the theory of quantum physics inside out, and we perform thousands of experiments that verify its predictions, there are still questions that remain. These questions involve the meaning, the interpretation of quantum physics. For example, if we can't know the position or momentum of a particle, does the particle even have a specific value of position or momentum? Or does the particle only have these attributes when we measure them? The surprising answers provided by quantum physics seem to be: no and yes. To illustrate this, let's go back again to Young's two slit experiment.
The
Two Slit Experiment Rerevisited
Remember, it goes like this. Young shone light through two very narrow slits, very close together, onto a screen. The light wave emerging from one slit interfered with the wave emerging from the other slit, and the result was not two narrow stripes of light (one for each slit) but a whole series of stripes that we call an interference pattern. Now let's consider this in terms of photons, particles of light. You might expect it to work as follows. We shine the photons at the slits, some go through one slit, some through the other. The photons emerging from one slit interfere with the photons emerging from the other, and we get an interference pattern. Now suppose we make the light very dim, and replace the screen we use to observe the result with a photographic film, thus giving us a permanent record of the light striking the screen. Dimmer light means fewer photons, and if the light's dim enough we can actually see the impact of the individual photons on the film. Instead of stripes, we get very tiny spots where the photons hit, but the spots arrange themselves into the interference pattern. That is, the places where we would have bright stripes if the light were brighter, are the places we see most of the photons hitting. Where the dark stripes would be, we get very few photons. One can conceivably make the light so dim that photons pass through the slits one at a time. Physicists have actually done this! And as the spots accumulate on the film, the interference pattern appears.
And
there's the problem. If we send just one photon at the slits, and it passes
through one slit or the other, what is there for it to interfere with when it
emerges? There must be interference because if we do this over and over again,
an interference pattern emerges. However, if the photon passes through one
slit, there's nothing coming out of the other slit!
The
only way to resolve this is to go back to wave thinking again. The waveparticle
passes through both slits, and the result that emerges from each slit
interferes with the result from the other slit. It's more than simply saying we
don't know which slit the photon passes through. The photon doesn't
pass through just one slit at all. In other words, as the photon passes through
the slits, not only don't we know it's location, it doesn't even have a
location. It doesn't have a location until we observe it on the film. This
paradox is the heart of what has come to be called the Copenhagen
interpretation of quantum physics.
The
Copenhagen Interpretation
Now I should start out here with a disclaimer. Nobody has ever really, as far as I know, set down an "official" version of the Copenhagen interpretation of quantum physics. If you ask ten different physicists what the Copenhagen interpretation is, you'll get nine similar (but not exactly the same) answers, and one "Who cares?" The interpretation of quantum physics is a bit out of the mainstream of physics education, because students are too busy learning how to calculate stuff. So what I'm going to call the Copenhagen interpretation is really just my interpretation of the Copenhagen interpretation. The situation's a bit unfortunate, but there it is.
The
Copenhagen interpretation concerns how much we can know about a particle, and
when. One of the central features of quantum physics, relative to this, is called
the "collapse of the wave function." (When I say "wave
function", just think "wave." Physicsist use the phrase
"wave function" because it sounds more technical. :-) In the two slit
experiment, after the wave-particle passes through the slits, we have a wave
that is spread out over a broad region, meaning that the photon could be
discovered anywhere. When it hits the film and makes a spot, this is no longer
the case. We now have the location of the photon narrowed down to a very small
region, which means that its wave function must be restricted to that region
also. A sudden change like this in a wave, due to a measurement (in this case,
the film "measures" the location of the photon), is the collapse of
the wave function. The exact mechanism behind this collapse is not really
known, and in my opinion the collapse of a wave function is the central mystery
of quantum physics.
I'll
summarize the Copenhagen interpretation of quantum physics this way:
- The wave function is a complete description of a wave-particle. That is, any information that can't be derived from the wave function doesn't exist. For example, if the wave is spread out over a broad region, then we can't determine where the particle is located. And since the wave function doesn't tell us the location, the particle doesn't have a location. Similarly, if a wave is made up of many different momenta, then the wave-particle doesn't have a value for the momentum.
- When a measurement of a wave-particle is made, its wave function collapses. So for example, if we precisely measure the momentum of a particle, its wave function suddenly changes from a wave made up of many momenta, to a wave with only one momentum. (This is termed a collapse, even though the wave doesn't actually get any smaller in this case. Remember, a wave with an exact momentum extends across the entire universe.)
- If two properties of a wave-particle are related by an uncertainty relation (such as the Heisenberg uncertainty principle), no measurement can simultaneously determine both properties to a precision greater than the uncertainty relation allows. In the case of the Heisenberg uncertainty principle, this means that if we measure the position of a particle, then there's a limit to the precision with which we can know the momentum. A consequence of this is that when we measure the position of a particle, we affect its momentum, and vice versa.
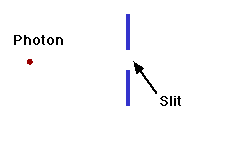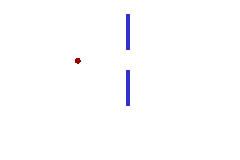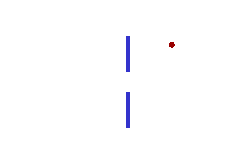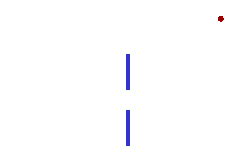
We can illustrate this last tenet of the Copenhagen interpretation, again with light passing through a narrow slit. The wave property of light dictates that when light passes through a slit, it doesn't travel straight through, but spreads out as it emerges from the slit.
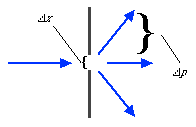
If we imagine a single photon passing through the slit, the slit "measures" the position of the photon. (That is, as the photon passes through the slit, its location must be somewhere within the region denoted Dx in the picture. This measurement of the position of the photon introduces an uncertainty in the momentum of the photon, reflected in the fact that the photon is no longer moving straight ahead, but is moving at an unknown angle. The uncertainty in momentum is labeled Dp in the diagram, and Dx Dp must be large enough to satisfy the Heisenberg uncertainty principle.
The
Bohr-Einstein Debate
The Copenhagen interpretation derives its name from its leading exponent, the Danish (hence "Copenhagen") physicist Niels Bohr. Bohr, among his many contributions to quantum physics, was the central figure in clarifying the implications of quantum physics in the early part of the twentieth century. He did this largely in response to a series of challenges by quantum skeptic (despite his role in the creation of the theory) Albert Einstein. These challenges, and Bohr's responses, are collectively referred to as the "Bohr-Einstein Debate."
This
was not a debate with two podia and a moderator. It was just an informal give
and take over the course of several meetings between Bohr and Einstein. A typical
encounter would consist of Einstein devising a hypothetical experiment for
precisely measuring both the position and momentum of a particle at a
particular moment, in violation of the uncertainty principle. Bohr would then
examine the experiment in more detail, and discover that there was no
inconsistency to the uncertainty principle after all.
An
example may prove instructive. I don't think this is actually an argument
brought up by Einstein, but it's close to one of his, and it's in the same
spirit. It involves a photon and a slit (you're probably getting sick of this
by now, but photons and slits are so useful in explaining quantum
physics). The slit has a width Dx, and so it measures the position of the
photon to that accuracy. The difference here is that the slit is allowed to
slide freely back and forth. The measurement of the position of the photon by
the slit alters the momentum of the photon in the direction parallel to the
width of the slit by an amount consistent with the uncertainty principle:
![]() .
.
But
there's another law of physics called the Law of Conservation of Momentum. In
our case, this law implies that if the momentum of the photon changes due to
passing through the slit, the momentum of the slit changes by an exactly
opposite amount (i.e. it recoils).
So
if we measure the recoil momentum of the movable slit, we get a measurement of
the momentum of the photon. And in principle, we can make the slit smaller and
smaller, until eventually the uncertainty principle is violated.
The
problem here is that we've applied the uncertainty principle only to the photon,
and not to the slit. We are measuring the momentum of the photon parallel to
the width of the slit by measuring the momentum change of the slit. This can
only work if we know what the momentum of the slit is to start with, and
we know it at least as precisely as we're trying to measure. Hence, the
uncertainty in the momentum of the slit as the photon passes through must
satisfy
![]()
And
the slit must satisfy the Heisenberg uncertainty principle, which means there's
an uncertainty in the position of the slit which satisfies
![]()
And
finally, since the only thing we know about the location of the photon is that
it passes through the slit, any uncertainty in the location of the slit is an
uncertainty in the location of the photon:
![]()
We
can then deduce from these three equations that
![]()
And
we see that this measurement is fully consistent with the Heisenberg
uncertainty principle! This is how the Bohr-Einstein debate went. Each time
Einstein proposed an experiment to beat the uncertainty principle, Bohr managed
to show that there was no inconsistency after all. In the end, Einstein was
forced to concede that quantum physics was at least consistent. (That's right,
Einstein lost the Bohr-Einstein debate!)
And so, as strange as its consequences may seem, quantum physics, including the Heisenberg uncertainty principle, stands up under the most thorough theoretical scrutiny (such as the Bohr-Einstein debate) and experimental scrutiny (a whole other story, perhaps for another time) that physicists have been able to muster. In the end, we are left with uncertainty.