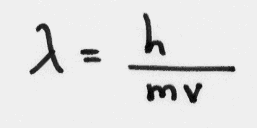
Louis de Broglie (1892 - 1987)
If light can be modeled as a particle or as a wave, can an electron be modeled as a wave?
The wavelength of a matter wave (1923) is given by:
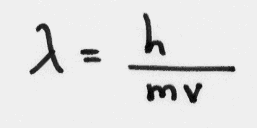
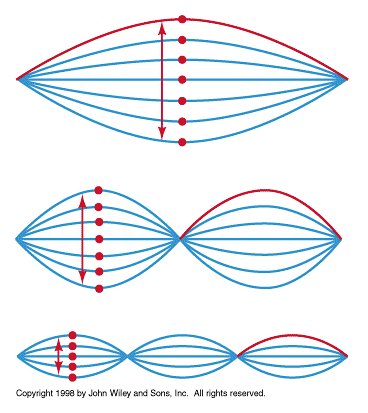
Everyday objects are too massive to give observable wavelengths; however, electrons are light enough to give observable wavelengths. Diffraction of electrons was observed by two groups in 1927, Davisson & Germer and George Thomson.
The Bohr model could also be explained using standing waves.
Whole numbers (1,2,3,etc.) of de Broglie wavelength give the allowed radii found in the Bohr model.