Schrodinger (1887 - 1961)
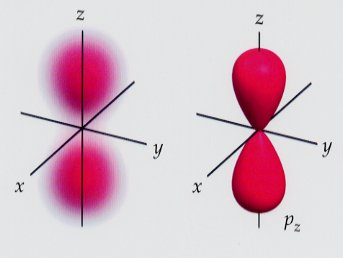
In 1926 Schrodinger applied a wave model to the one electron in the hydrogen atom. The solution to his wave equation was a series of wave functions. Each wave function provided information on the location and energy of the electron in a possible orbital.The wave function squared gave a probability distribution for the electron in that orbital.

The energies of the allowed orbitals are shown below.
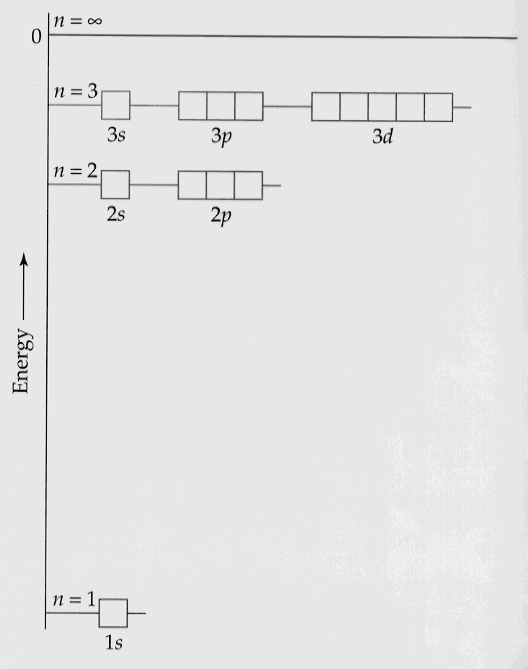
Note the similarity to the Bohr model.