
Heisenberg (1901 - 1976)
Note: Heisenberg also solved the wave equation for the hydrogen atom in 1926; he used matrix algebra instead of calculus to arrive at the solution.

In 1927 he proposed the Uncertainty Principle, which describes an irreducible level of uncertainty in measurements of position and momentum of electrons. Mathematically,
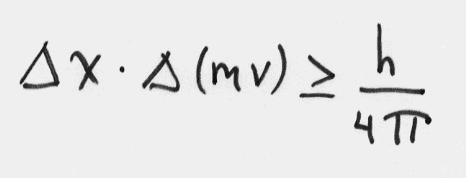
One can be certain about position or momentum of an electron, but not both at the same time. For example, if the uncertainty in momentum is 1%, the uncertainty in position is six times the size of hydrogen atom! This limitation is inherent in nature; there is no way to overcome it. This is radically different than our everyday world.
Any measurement on the atomic scale significantly alters the object being measured.