Quantum Mechanics
Although the wave equation can only be solved exactly in the one electron case, Schrodinger's approach was extended to atoms (and eventually molecules) containing many electrons. This area of research is called Quantum Mechanics.
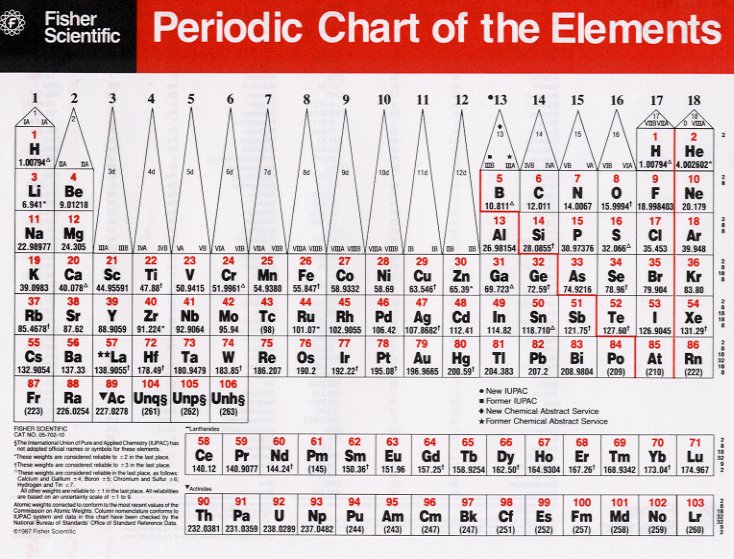
The energies of the allowed orbitals in multi-electron atoms are shown below.

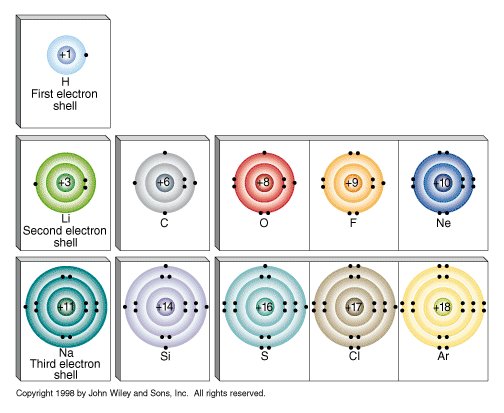
Each box represents an orbital, which can hold a maximum of two electrons. Filling from bottom to top with the correct number of electrons gives the electron configuration of an atom. This scheme explains the periods(rows) and groups(columns) in the periodic table.